WEB TUTORIAL - Group Theory
[Re2Cl8]2- Worked Example
| D4h | E | 2C4 (z) | C2 | 2C'2 | 2C''2 | i | 2S4 | 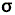 h h |
2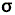 v v |
2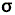 d d |
Rotations |
Functions |
Functions |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1g | +1 | +1 | +1 | +1 | +1 | +1 | +1 | +1 | +1 | +1 | - | x2+y2, z2 | - |
| A2g | +1 | +1 | +1 | -1 | -1 | +1 | +1 | +1 | -1 | -1 | Rz | - | - |
| B1g | +1 | -1 | +1 | +1 | -1 | +1 | -1 | +1 | +1 | -1 | - | x2-y2 | - |
| B2g | +1 | -1 | +1 | -1 | +1 | +1 | -1 | +1 | -1 | +1 | - | xy | - |
| Eg | +2 | 0 | -2 | 0 | 0 | +2 | 0 | -2 | 0 | 0 | (Rx, Ry) | (xz, yz) | - |
| A1u | +1 | +1 | +1 | +1 | +1 | -1 | -1 | -1 | -1 | -1 | - | - | - |
| A2u | +1 | +1 | +1 | -1 | -1 | -1 | -1 | -1 | +1 | +1 | z | - | z3, z(x2+y2) |
| B1u | +1 | -1 | +1 | +1 | -1 | -1 | +1 | -1 | -1 | +1 | - | - | xyz |
| B2u | +1 | -1 | +1 | -1 | +1 | -1 | +1 | -1 | +1 | -1 | - | - | z(x2-y2) |
| Eu | +2 | 0 | -2 | 0 | 0 | -2 | 0 | +2 | 0 | 0 | (x, y) | - | (xz2, yz2) (xy2, x2y), (x3, y3) |
h = 16
Consider each symmetry element in turn, and answer how many unchanged bonds there are in each case after the symmetry element has been completed. This example is quite tricky but it will help to make a model, or to use the visualiser on Otterbein Website.
Help
One way to help work out the number of unchanged bonds is to label them. The bonds have been labelled in [Re2Cl8]2- for the C2' rotation.
Some labels are shown on the atoms to make them easier to track.
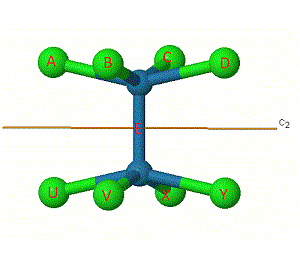
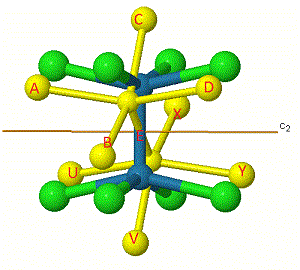
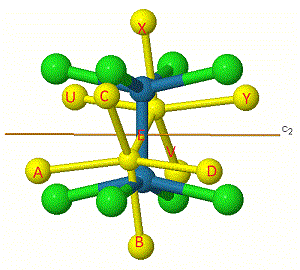
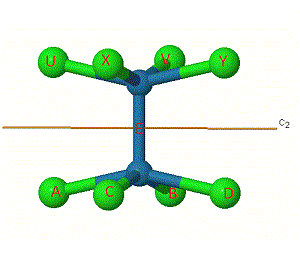
The same principle applies to i, S4, and ![]() h symmetry elements, which at first appear to change the position of the 'middle' bond E, but infact this stays as an unchanged bond.
h symmetry elements, which at first appear to change the position of the 'middle' bond E, but infact this stays as an unchanged bond.
The first number indicates the number of unchanged bonds for E, the second number
indicates the number of unchanged bonds for the C4 rotation and so forth.
-
E 2C4 C2 2C'2 2C"2 i 2S4
- 9 1 1 1 1 1 1 1 5 1
- 9 1 1 0 0 0 0 0 5 1
- 9 1 1 0 0 1 1 0 5 1